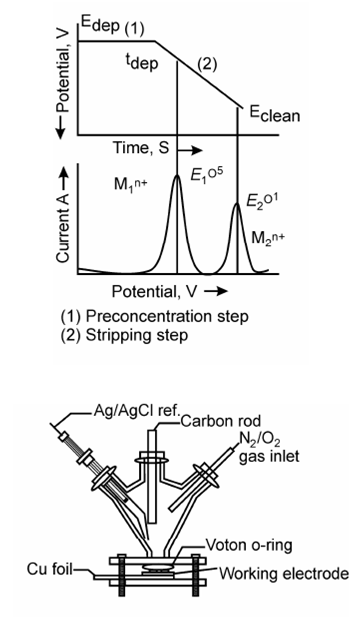
Details of stripping voltammetry:
In anodic stripping voltammetry the determination of the analyte is based on the anodic dissolution. This procedure is followed voltammetrically and the peak current thus obtained is proportional to the potential scan rate and radius of mercury drop (HMDE) or scan rate and surface area of the mercury film (TMFE). Within both the cases the peak current ip is given through
ip = K. 3/2n.D1/2cM0(Hg) υ1/2 r 2 .tace
ip = K.n2 .A pc M 0 (Hg) υ tace
Where, ip = peak current, n = electron transfer during the oxidation of the analyte, D = diffusion coefficient, K = constant, CM° = concentration of metal in the amalgam, AF = surface area, υ = scan rate, and tace = accumulation time.
Therefore in both the cases the peak current depends on the accumulation time tace and therefore, is proportional to the concentration of analyte cM° (Hg) in the amalgam and indirectly proportional to the concentration of the analyte in the sample solution.
Peak currents in this method are a linear function of concentration and hence this technique can be used for the determination of many metal ions in trace quantities in environmental samples.