Shielding of acetylenic protons:
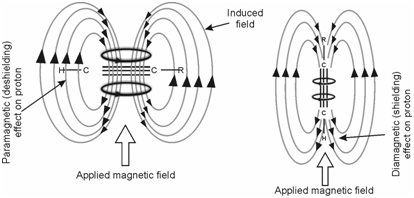
In case of alkynes or acetylenic protons, therefore, the protons appear upfield in the range 1.5 to 3.5. Electron circulation around the triple bond occurs in such a way that protons experience a diamagnetic shielding effect as shown in Figure. In this case, it is essential to consider the parallel as well as perpendicular orientations of acetylene molecule. If the axis of acetylene molecule is aligned parallel to the applied magnetic field B0 as shown in Figure (b), the π-electrons in the triple bond readily induce diagmagnetic circulation and the magnetic field so generated opposes the applied field at the acelytenic protons. This is further to the local diamagnetic shielding effects experienced through protons. Therefore, if the axis of acetylene molecule is perpendicular to the applied field as shown in Figure (a) then π-electron circulation is severely restricted and as can be seen from the figure, the induced field augments the applied field at the acelytenic protons. It is since of this pronounced anisotropy of the triple bond, the additional shielding, experienced by the protons varies considerably with the orientation. The net effect of the two effects is that the diamagnetic anisotropy of the triple bond predominates and serves to increase the shielding of the acetylenic protons. This explains the high field shift of the acetylenic protons.
(a) (b)
Figure: Shielding of acetylenic protons by triple bond in (a) perpendicular to the applied field and (b) parallel orientation