Deshielding of aromatic protons:
In aromatic rings like as benzene, a various type of diamagnetic shielding is observed due to the unsymmetrical distribution of π-electrons. These are readily induced into circulation in the plane of the ring by the applied magnetic field. Figure shows the secondary magnetic field generated by the induced circulation of π-electrons in benzene molecule aligned perpendicular to the applied field. The effects of the secondary magnetic field on a rigidly attached proton in the molecule do not average to zero for all possible orientations of the ring with respect to the applied field. You may note here that secondary magnetic field causes pronounced shielding at the centre of the ring but deshielding outside, in the plane of the ring containing protons.
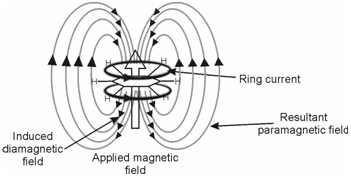
Figure: Deshielding of aromatic protons due to induced magnetic field
Due to the pronounced deshielding the δ value for benzene protons is observed at 7.22 ppm, however, it gets shifted to lower or higher side in the range 6.5 to 8.5 ppm, depending on the nature of substituent, in the benzene derivatives. In fact a chemical shift value within this range is an indicator of the presence of benzene or aromatic compounds.
Hydrogen bonds: In case of hydrogen bonding, the H-atom is shared through two electronegative atoms. That is highly deshielded and the resonance occurs at low field and correspondingly high δ value is observed. All alcohols, amines and thiols show varying degree of H-bonding.