Rearrangement of a primary carbocation to a tertiary carbocation:
For a straight chain alkane the characteristic peaks are 14 mass units apart, but this does not indicate that the chain is being 'pruned' one methylene unit at a time. Decomposition of carbocations takes place with the loss of neutral molecules like methane, ethene and propene, and not by the loss of individual methylene units. For instance, the daughter ion at m/e 99 can fragment with loss of propene to provide the ion at m/e 57. The daughter ion at m/e 85 can fragment with loss of ethene or propene to provide the ions at m/e 57 and m/e 43 correspondingly. The daughter ion at m/e 71 can fragment with loss of ethene to provide the ion at m/e 43.
There are major peaks at m/e 27 and m/e 41. These peaks effect from dehydrogenation of the ions at m/e 29 and m/e 43 correspondingly. The peak at m/e 41 can as well arise from the ion at m/e 57 by loss of methane. The several intense peaks in the mass spectrum are at m/e 43 and m/e 57. The ions accountable for these peaks [C3H7]+ and [C4H9]+ can take place from primary fragmentations of the molecular ion itself, also from secondary fragmentations of daughter ions (m/e 99 to m/e 57; m/e 85 to m/e 43; m/e 71 to m/e 43).
In mass spectroscopy, the ions accountable for specific peaks are enclosed in square brackets. This is since it is not really possible to identify the exact structure of an ion or the exact location of the charge. The ionization conditions employed in mass spectroscopy are such that fragmentation ions can simply rearrange to form structures much more capable of stabilizing the positive charge. For instance, the fragmentation ion at m/e 57 arising from primary fragmentation is a primary carbocation, but this can rearrange to the much more stable tertiary carbocation.
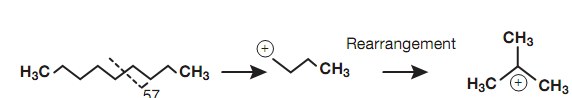
Figure: Rearrangement of a primary carbocation to a tertiary carbocation.