Chemiluminescence method:
As [M] ∝ pressure employed, at low pressures,
I = d(photons)/dt ∝[O3][NO]
At the operating pressure of 0.01 - 0.05 atmospheres the intensity of a signal is quite good and the extent of signal from PMT is proportional to concentration of NO and the concentrations as little as 1 ppb of the gas could be measured.
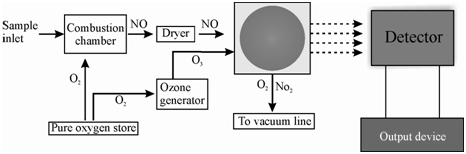
Figure: Schematic layout for the determination of NO-NO2 by chemiluminescence method
In sequence to determine the amount of nitrogen dioxide, NO2, in a sample having no nitric oxide, NO, that is first converted to nitric oxide, NO, by passing through a catalytic converter. Thereafter, it is passed through the reactor for activation by ozone as discussed. The photon count from the PMT is proportional to the concentration of NO which is a measure of NO2 before it was converted to NO.
In the sample holding a mixture of NO and NO2, the sample is passed by the reactor and the amount of NO is determined. Thereafter, within a separate experiment the sample is passed by the converter (so as to convert the NO2 to NO) before sending to the reactor. The photon count in like a case provides the total amount of NO and NO2 in the air sample. The amount of NO2 is acquired through subtracting the value for NO from that of the mixture.