Analysis of microgram concentration of pollutants:
The fluorescence occurs at low pressure (0.5 atm). A fluorescence signal is detected through a photomultiplier tube and is properly recorded. One can measure 0.5 - 1000 ppm of SO2 by fluorimetry.
The reaction along with para-rosaniline and formaldehyde could be shown as below.
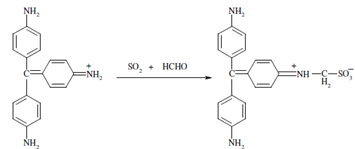
p - Rosaniline p - Rosanilinemethylsulphonic acid
The other method, based on chemiluminescence is even more reliable, accurate and less time consuming. Within this method, the sample holding atmospheric sulphur compounds such as, sulphur dioxide, hydrogen sulphide and merceptans, etc. is combusted within the hydrogen flame. This produced a sulphur dimer which decomposes along with the emission of characteristic blue light with λmax at 384 nm and 394 nm. One could detect as small as 5 ppb of SO2 gas through this method; the reactions for SO2 gas are as follows:
4H2 + 2SO2 ↔ S*2 +4H2
S*2 → S2 +hv
The chemiluminescence technique is rapid and allows analysis of microgram concentration of pollutants. The methods have been commercialised and one could buy automated analysers from the market. The method is free from interferences through CO2, CO, SO2, O3, hydrocarbons and water vapour. It gives continuous measuring devise. As regards the limitations the problem area is that all nitrogen containing compounds generates NO2 on combustion. It is significant to know concentration ranges of SO2 as well as NO2 in the atmosphere.