Reactions of Amines:
Alkylation
Ammonia, primary amines and secondary amines (both of the aromatic and aliphatic) can go through the SN2 reaction with alkyl halides to generate a range of primary, secondary, and tertiary amines. Primary, secondary, and tertiary amines are produced like ammonium salts that are transformed to the free amine through treatment with sodium hydroxide as shown in figure.
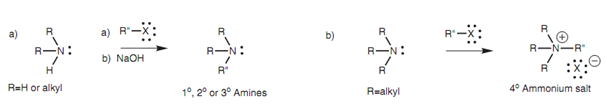
Figure: Alkylation of amines.
Theoretically, it should be probable to synthesize primary amines from ammonia, secondary amines from primary amines, and third one the tertiary amines from secondary amines. Practically, over-alkylation is general. For instance, reaction of ammonia with methyl iodide directs to a mixture of primary, secondary, and tertiary amines together with a small quantity of the quaternary ammonium salt as described in picture.
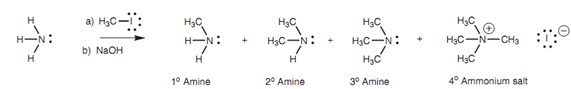
Figure: Alkylation of ammonia with methyl iodide.
Alkylation of tertiary amines through this method is a good way of acquiring quaternary ammonium salts because no other products are possible. Though, alkylation of lower order amines is not that much satisfactory. A good method of alkylating a primary or secondary amine is to treat the amine with a ketone or an aldehyde in the existence of a reducing agent - sodium cyanoborohydride. This reaction is termed as reductive amination. Over-alkylation cannot take place via this method.