Decarboxylation mechanism:
A further point worth noting is that the ethoxide ion is sufficiently strong to deprotonate the diethyl malonate quantitatively like that all the diethyl malonate is transformed to the enolate ion. This stops the probability of any competing Claisen reaction (see below) because that reaction needs the existence of unaltered ester. Diethyl malonate can be transformed quantitatively to its enolate along with ethoxide ion, alkylated with an alkyl halide, treated with other equivalent of base, and after that alkylated with a second different alkyl halide. Subsequent hydrolysis and decarboxylation of the diethyl ester causes in the creation of the carboxylic acid. The decarboxylation mechanism shown in figure is dependent on the existence of the other carbonyl group at the β-position.
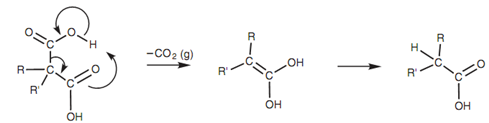
Figure: Decarboxylation mechanism.
The last product can be viewed like a disubstituted ethanoic acid. Theoretically, this product could as well be synthesized from ethyl ethanoate. Though, the make use of diethyl malonate is superior because the presence of two carbonyl groups permits easier formation of the intermediate enolate ions.