Organometallic Reactions:
Grignard reagents
Alkyl halides of all types such as (1?, 2? and 3?) react with magnesium in dry ether to make Grignard reagents, in which the magnesium is 'inserted' among the halogen and the alkyl chain as shown in figure.
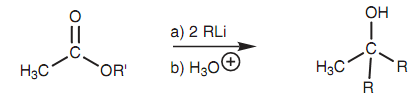
Figure: Formation of a Grignard reagent (X= Cl, Br, I).
These reagents are very useful in organic synthesis and can be employed in a wide range of reactions. Their reactivity reflects the polarity of the atoms that are present. Because magnesium is a metal it is electropositive that means that the electrons in the C-Mg bond spend much more of their time closer to the carbon creating it slightly negative and a nucleophilic center. This reverses the character of this carbon because it is an electrophilic center in the original alkyl halide. Basically, a Grignard reagent can be viewed as providing the equivalent of a carbanion. The carbanion is not a different species; however the reactions which occur can be described as if it was.
Figure: Conversion of a Grignard reagent to an alkane.