Reduction of Alkynes:
Hydrogenation
Alkynes react along with hydrogen gas in the existence of a metal catalyst in a process termed as hydrogenation - an instance of a reduction reaction. With a fully active catalyst like platinum metal, two molecules of hydrogen are added to generate an alkane.
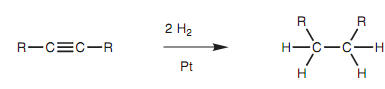
Figure: Reduction of an alkyne to an alkane.
The reaction includes the addition of one molecule of hydrogen to create an alkene intermediate that then reacts with a second molecule of hydrogen to create the alkane. With less active catalysts, it is feasible to stop the reaction at the alkene stage. Particularly, Z-alkenes can be synthesized from alkynes through reaction with hydrogen gas and Lindlar's catalyst as shown in figure. This catalyst contains metallic palladium deposited on calcium carbonate that is then treated with lead acetate and quinoline. The latter treatment 'poisons' the catalyst like that the alkyne reacts with hydrogen to provide an alkene, but does not react further. Because the starting materials are absorbed onto the catalytic surface, both hydrogens are added to similar side of the molecule to generate the Z isomer.
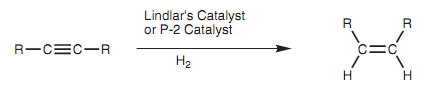
Figure: Reduction of an alkyne to a (Z)-alkene.
An alternative catalyst that acquires similar result is nickel boride (Ni2B) - the P-2 catalyst.