Properties of Alkenes And Alkynes:
Structure
Alkene functional group (R2C=CR2) is planar in shape along with bond angles of 120?. The two carbon atoms that are involved in the double bond are both sp2 hybridized. Every carbon has three sp2 hybridized orbitals that are employed for σ bonds while the p orbital is employed for a π bond. So, the double bond is made up of one σ bond and one π bond.
The alkyne functional group contains a carbon carbon triple bond and is linear in shape along with bond angles of 180?. The two carbon atoms included in the triple bond are both sp hybridized like that each carbon atom has two sp hybridized orbitals and two p orbitals. The sp hybridized orbitals are employed for two σ bonds whereas the p orbitals are employed for two π bond s. So, the triple bond is made up of one σ bond and two π bonds.
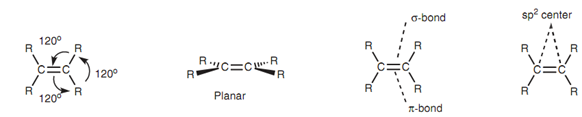
Figure: Structure of an alkene functional group.
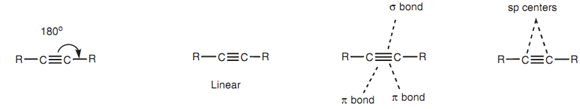
Figure: Structure of an alkyne functional group.