Electrophilic Addition to Unsymmetrical Alkenes:
Addition of hydrogen halides
The reaction of a symmetrical alkene along with hydrogen bromide generates similar product regardless of whether the hydrogen of HBr is added to one end of the double bond or another. Though, this is not the case with unsymmetrical alkenes shown as figure. In this case, two dissimilar products are possible.
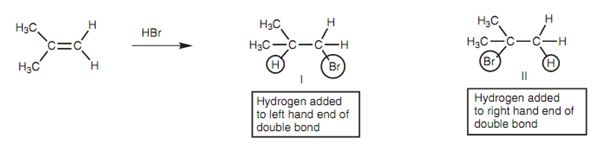
Figure: Electrophilic addition of HBr to an unsymmetrical alkene.
These are not created to an equal extent and the much more substituted alkyl halide (II) is preferred. The reaction carries on in a Markovnikov sense with hydrogen ending up on the least substituted position and the halogen ending up on the most substituted position.
Markovnikov's rule says that 'in the addition of HX to an alkene, the hydrogen atom adds to the carbon atom which already has the greater number of hydrogen atoms'. This generates the more substituted alkyl halide.