Newman projections of the staggered:
Viewed in this manner, it can be observed that the C-H bonds on carbon 1 are eclipsed along with the C-H bonds on carbon 2 in conformation II.
By these two conformations, the staggered conformation is the more stable because the C-H bonds and hydrogen atoms are as far except each other as possible. In the eclipsed conformation, both the bonds and the atoms are much closer together and this can cause strain because of electron repulsion among the eclipsed bonds and among the eclipsed atoms. Hence, the vast majority of ethane molecules are in the staggered conformation at any one time. Though, it is significant to realize that the energy variations among the staggered and eclipsed conformations is still sufficiently small to permit each ethane molecule to pass through an eclipsed con- formation - otherwise C-C bond rotation would not take place.
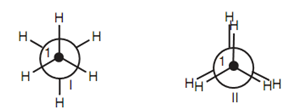
Figure: Newman projections of the staggered (I) and eclipsed (II) conformations of ethane.
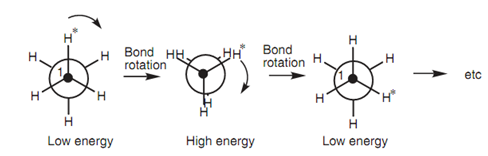
Figure: Bond rotation of ethane.