Gauche conformation:
Ethane has just only one type of staggered conformation, but diverse staggered conformations are possible with larger molecules like butane.
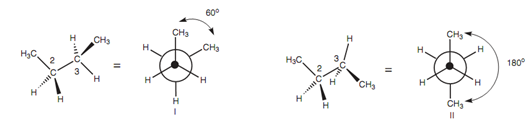
Figure: Gauche conformation (I) and anti conformation (II) of butane.
The 1st and the 3rd C-C bonds in isomer I are at an angle of 60? with respect to each other while viewed together the middle C-C bond. In isomer II, these bonds are at an angle of 180?. This angle is termed as the torsional angle / dihedral angle. Isomer II is more stable as compared to the isomer I. This is since the methyl groups and the C-C bonds in this conformer are as far except for each other as possible. The methyl groups are bulky and in conformation I they are sufficiently close to interact with each other and direct to some strain. There is as well an interaction among the C-C bonds in isomer I because a torsional angle of 60? is small enough for a number of electronic repulsion to exist among the C-C bonds. While C-C bonds comprise a torsional angle of 60?, the steric and electronic repulsions that arise are considered to as a gauche interaction.