Solution and coordination chemistry
The properties of the M2+ aqueous ions depict trends supposed from their increasing size down the group. Be2+ (like Al3 +) is amphoteric. The insoluble hydroxide dissolves in both acid solution:
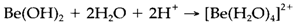
and in alkaline conditions:
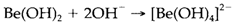
The simple aqua cation is exists only in strongly acidic circumstances. Like the pH increases, successive polymerization and protolysis reactions first provide soluble species with Be-OH-Be bridges, and then the solid hydroxide. Other M2+ ions are basic. Like the hydroxide M(OH)2 becomes more soluble in the series Mg<Ca <Sr<Ba precipitation needs increasingly high pH. Complex formation is dominated through class a or 'hard' behavior and is usually most favorable for the smaller ions. Beryllium forms [BeF4]2- and strong complexes with some bidentate ligands like oxalate C2O42- . From carboxylic acids not usual complexes like [Be4O(O2CCH3)6] can be acquired; the structure (1) has a central oxygen atom surrounded via a Be4 tetrahedron with acetate groups bridging the edges. The larger ions creates complexes with chelating ligands like EDTA. Complexes with ammonia like [Mg(NH3)6]2+ can be prepared in nonaqueous conditions but are not stable in water. Though, chlorophylls, that are necessary for photosynthesis in all green plants, have magnesium coordinated through nitrogen in macrocyclic porphine derivatives: 2 depicts the basic framework, that has other organic groups attached; Mg2+ generally has one water molecule also coordinated.
