Organometallic compounds
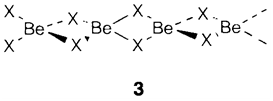
Be and Mg create an extensive variety of organometallic compounds, those of Ca, Sr and Ba being very much reactive and hard to characterize. Beryllium alkyls like Be(CH3)2 comprise chain structures (see 3 with X=CH3) with multicenter bonding identical to that in Li4(CH3)4 and Al2(CH3)6. Be and Mg create biscyclopentadienyl compounds M(C5H5)2; the Mg compound has an η5 sandwich structure as of ferrocene but is much more reactive and at least partially ionic: M2+(C5H5-)2. The Be compound is less symmetrical with one ring displaced sideways, most probably due to the small size of Be.
Certainly the most generally encountered organometallic compounds in group 2 are the Grignard reagents RMgX, created through reaction of Mg metal with an alkyl or aryl halide RX in an ether solvent. Solid compounds along with additional ether molecules coordinated to Mg can be acquired, but the reagents are usually used in solution. They are very helpful for arylation and alkylation reactions, either for creating C-C bonds in organic chemistry or for preparing organometallic compounds of another element.