Solid compounds
The alkali metals react with several other elements straight to make binary solids. The alkali halides are often considered as the most 'typical' ionic solids. Their lattice energies agree directly with calculations even though their structures do not all conform to the simple radius ratio rules, as all contain the rocksalt (NaCl) structure at usual temperature and pressure, apart from CsCl, CsBr and CsI, that have the eight-coordinate CsCl structure. The alkali halides are all fairly soluble in water, LiF being the smallest so.
The elements also create hydrides through direct interaction among the elements. LiH is the very stable and is a helpful precursor for other hydrides. With N2 to create the nitride Li3N lithium also reacts.
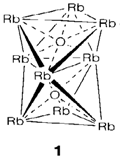
The elements creates oxides M2O, that have the antifluorite structure for Li-Rb. Cs2O has the extremely unusual anti- CdI2 structure with adjacent layers of Cs-. All compounds are especially basic and react with water and CO2 to make carbonates and hydroxides, correspondingly. Though, apart from for Li, the simple oxides are not the normal results of burning the elements in air. K, Rb and Cs create superoxides MO2 consisting of the O2- ion, and sodium the peroxide Na2O2 with O22-. The comparative stability of these compounds with O2-large cations of low charge can be understood through lattice energy arguments. Rb and Cs also creates suboxides when oxygen supply is extremely deficient, for instance, Rb9O2 (1) and Cs11O3; the former compound's structure is based on two facesharing octahedra with direct Rb-Rb bonding giving distances shorter than in the metallic element.
Hydroxides MOH are very significant compounds for all the alkali metals, being simply formed through reaction of oxides with water (or atmospheric moisture), and soluble in water providing classic strong base behavior. Compounds of oxoacids are generally encountered, like carbonate, nitrate, sulfate, etc. As these anions are quite large, lithium compounds tend to be the main soluble in the series. Several of these compounds crystallize in a range of hydrated forms (example Na2CO3.nH2O with n=1, 7 or 10).
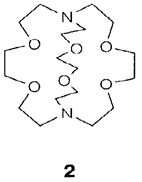
The mixture of the reducing power of alkali metal-ammonia solutions with the strong complexing power of macrocyclic ligands permits compounds to be made consisting of not usual anions, like [Sn9]4-. Between the unexpected products of such type of reactions are alkalide and electride salts. An instance of an alkalide is [Na(2. 2.2.crypt)]+Na-, in which crypt is the cryptand ligand 2. The crystal structure depicts that the Na- ion is larger than I-. In electrides like [Cs(18-crown-6)2]+e- there is a 'bare' electron trapped within a cavity in the lattice.