Properties:
Because of the polar nature of the carbonyl group, aldehydes and ketones comprise higher boiling points as compared to the alkanes of similar molecular weight. Though, hydrogen bonding is not possible among the carbonyl groups and thus aldehydes and ketones have lower boiling points as compared to the alcohols or carboxylic acids. Low molecular weight aldehydes and ketones (for example formaldehyde and acetone) are soluble in water. This is since the oxygen of the carbonyl group can take part in intermolecular hydrogen bonding along with water molecules.
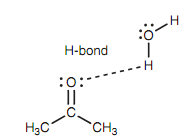
Figure: Intermolecular hydrogen bonding of a ketone with water.
Since molecular weight rises, the hydrophobic character of the attached alkyl chains begins to outweigh the water solubility of the carbonyl group along with the result that large molecular weight aldehydes and ketones are insoluble in water. Aromatic ketones and aldehydes are not soluble in water because of the hydrophobic aromatic ring.