Nucleophilic Addition - Oxygen and Sulfur Nucleophiles
Acetal and ketal formation
While an aldehyde or ketone is considered with an excess of alcohol in the existence of an acid catalyst, two molecules of alcohol are added to the carbonyl compound to provide an acetal or a ketal correspondingly. The last product is tetrahedral.
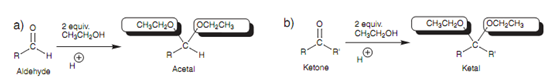
Figure: Formation of an acetal and a ketal.
The reaction mechanism includes the nucleophilic addition of one molecule of alcohol to make a hemiacetal or hemiketal. Elimination of water occurs to make an oxonium ion and a second molecule of alcohol is after that added.
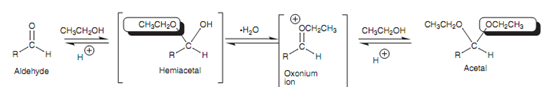
Figure: Acetal formation and intermediates involved.