Nucleophilic Addition - Charged Nucleophiles:
Carbanion addition
Carbanions are very reactive species and do not take place in isolation. Though, there are two reagents that can supply the equivalent of a carbanion. These are Grignard and organolithium reagents. We shall come across very first at the reaction of a Grignard reagent along with aldehydes and ketones.
The Grignard reagent in this reaction is termed as methyl magnesium iodide
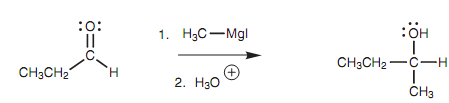
Figure: Grignard reaction.
(CH3MgI) and is the source of a methyl carbanion. In reality, the methyl carbanion is never exists as a separate ion, although the reaction proceeds as if it were. The methyl carbanion is the nucleophile in this type of reaction and the nucleophilic center is the negatively charged carbon atom. The aldehyde is the electrophile. Its electrophilic center is the carbonyl carbon atom because it is electron deficient.
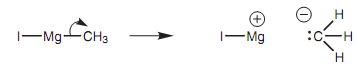
Figure: Grignard reagent.