Hydride addition:
Reducing agents like sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4) react along with aldehydes and ketones as if they are giving a hydride ion (:H-).
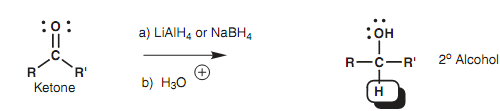
Figure: Reduction of a ketone to a secondary alcohol.
This species is not exists as such and the reaction mechanism is more complex. Though, we can describe the reaction by viewing these reagents like hydride equivalents (:H-). The complete reaction is an instance of a functional group transformation because the carbon skeleton is not affected. Aldehydes are transformed to primary alcohols and ketones are transformed to secondary alcohols. The mechanism of the reaction is similar as that explained above for the Grignard reaction.
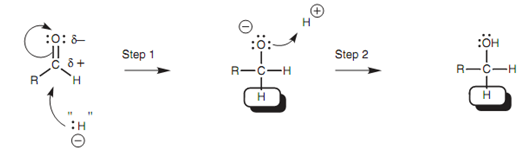
Figure: Mechanism for the reaction of a ketone with LiAIH4 or NaBH4.
The hydride ion equivalent adds to the carbonyl group and a negatively charged intermediate is acquired that is complexed like a lithium salt (Step 1). Consequent treatment with acid provides the final product (Step 2). It should be underlined once again that the mechanism is actually much more complex than this since the hydride ion is very much reactive to exist in isolation.