Electronic and Steric Effects:
Reactivity
Usually it is found that aldehydes are much more reactive to nucleophiles as compared to ketones. There are two factors (electronic and steric) that describe this variation in reactivity.
Electronic factors
The carbonyl carbon in aldehydes is much more electrophilic as compared to it is in ketones because of the substituents connected to the carbonyl carbon. A ketone comprises two alkyl groups attached while the aldehyde has just only one. The carbonyl carbon is electron deficient and electrophilic because the neighboring oxygen has a greater share of the electrons in the double bond. Though, neighboring alkyl groups comprises an inductive effect whereby they push electron density in the direction of the carbonyl carbon and make it less electrophilic and less reactive to nucleophiles.
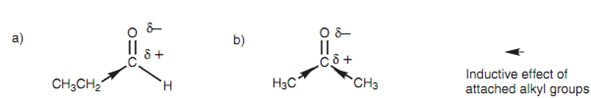
Figure: Inductive effect in (a) propanal; (b) propanone.