Reactions of Phenols:
Acid-base reactions
Phenols are much stronger acids than alcohols and react with bases like sodium hydroxide to form phenoxide ions. Though, they are weaker acids than carboxylic acids and do not react along with sodium hydrogen carbonate.
Phenols are acidic as the oxygen's lone pair of electrons can participate in a resonance mechanism including the adjacent aromatic ring as shown in figure.
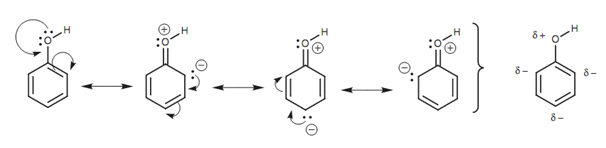
Figure: Resonance structures for phenol.
Three resonance structures are probable in which the oxygen gains a positive charge and the ring gains a negative charge. The complete result is a slightly positive charge on the oxygen that accounts for the acidity of its proton. There are as well three aromatic carbons with slightly negative charges.