Chemistry of Thiols:
Preparation
Thiols can be ready by the treatment of alkyl halides along with an excess of KOH and hydrogen sul?de. The preparation is an SN2 reaction including the generation of a hydrogen sul?de anion (HS-) like nucleophile. A difficulty with this reaction is the chance of the product being ionized and reacting with a second molecule of alkyl halide to generate a thioether (RSR) like a byproduct. An excess of hydrogen sul?de is generally employed to avoid this problem.
The problem of thioether formation can as well be avoided by using an alternative process involving thiourea. The thiourea works as the nucleophile in an SN2 reaction to generate an S-alkylisothiouronium salt that is then hydrolyzed with aqueous base to give the thiol.
Thiols can as well be formed by reducing disul?des with zinc in the existence of acid.
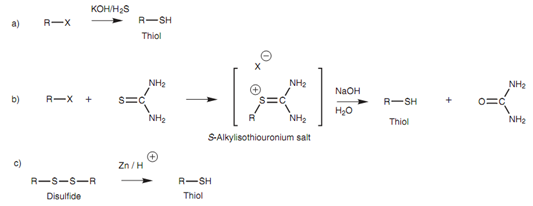
Figure: Synthesis of thiols.