Nitration of toluene:
An alkyl group can stabilize a neighboring positive charge through an inductive, electron-donating effect that results in some of the positive charge that is being spread over the alkyl group. This is an additional stabilizing effect that is only feasible for the intermediates taking place from ortho and para substitution. There is no such equal resonance structure for the meta intermediate and thus that means that the ortho and para intermediates experience an raised stability over the meta that results in a preference for these two substitution pathways.
By similar token, toluene will be much more reactive than benzene. The electron- donating effect of the methyl group in the aromatic ring creates the ring inherently much more nucleophilic and more reactive to electrophiles, also providing extra stabilization of the reaction intermediate. To summarize, alkyl groups are activating groups and are ortho, para directing.
The nitration of toluene as well demonstrates this effect. The quantity of Meta substitution is very small like we would suppose, and there is a preference for the ortho and para products. Though, why is there more ortho substitution as compared to para substitution? Quite basically, there are 2 ortho sites on the molecule to one para site and thus there is double the possibility of ortho attack to para attack. Based upon pure statistics we would have supposed the ratio of ortho to para attack to be 2:1. Actually, the ratio is closer to 1.5:1. Other words, there is less ortho substitution than supposed. This is since the ortho sites are directly 'next door' to the methyl substituent and the size of the substituent tends to interfere with ortho attack - a steric effect. The importance of this steric effect will change as per to the size of the alkyl substituent. The larger the substituent, the much more ortho attack will be hindered.
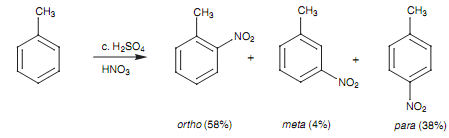
Figure: Nitration of toluene.