Bromination of aniline:
Suppose for instance we required to make para-bromoaniline through brominating aniline. In theory, this reaction scheme should provide the needed product. In practical, the NH2 group is such a strong activating group that the last bromination goes three times to provide the tri-brominated product than the mono-brominated product.
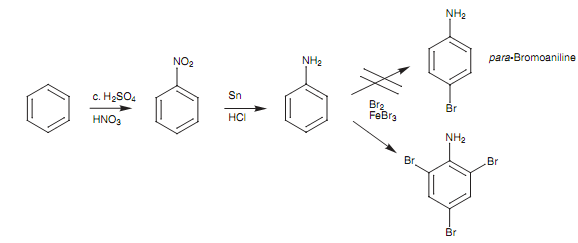
Figure: Bromination of aniline.
To lower the activation of the amino group, we can transform it to the much less activating amide group. After that the bromination only goes one time. We as well find that the bromination reaction is much more selective for the para position as compared for the ortho position. This is since the amide group is bulkier as compared to the NH2 group and tends to shield the ortho positions from attack. One time the bromination has been finished the amide can be converted back to the amino group through hydrolysis.
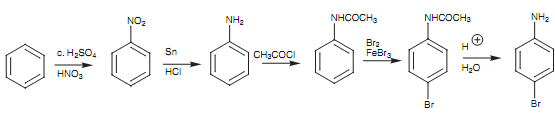
Figure: Synthesis of para-bromoaniline.