Amide resonance:
Because none of the resonance structures taking place from Meta attack locates the positive charge next to the phenol group, this fourth resonance structure is not exist to the meta intermediate and so meta attack is not favored. So, the phenol group is an activating group that is ortho, para directing due to resonance effects. This resonance effect is much more significant than any inductive effect that the oxygen might have.
The same holds true for the subsequent substituents: alkoxy (-OR), esters (-OCOR), amines (-NH2, -NHR, -NR2), and amides (-NHCOR). In all these types of cases, there is either oxygen or nitrogen next to the ring. Both of these atoms are nucleophilic and have lone pairs of electrons that can be employed to form an extra bond to the ring. The easiness with which the group can do this depends upon the nucleophilicity of the attached atom and how well it can handle with a positive charge.
Nitrogen is much more nucleophilic than oxygen because it is better able to handle with the resultant positive charge. Hence amine substituents are stronger activating groups as compared to ethers. Alternatively, an amide group is a weaker activating group because the nitrogen atom is less nucleophilic. This is since the nitrogen's lone pair of electrons is pulled towards the carbonyl group and is less likely to create a bond to the ring. This amides' property can be quite helpful.
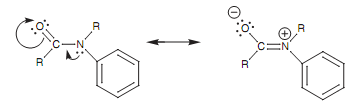
Figure: Amide resonance.