Nuclear properties
Following actinium (group 3) are the 14 elements of the actinide series (denoted through the symbol An) related with progressive filling of the 5f shell and thus similar to lanthanides. All are radioactive, its longest-lived isotopes being displayed in Table 1. The increasingly shorter half-lives reflect the falling stability of heavy nuclei, resultant from
the changing balance among the repulsive Coulomb forces and attractive strong interaction. Several actinide nuclei go through α decay through emitting 4He, although for heavier elements spontaneous fission into two fragments is an progressively more significant another decay route.
Only uranium and thorium contain half-lives long sufficient to survive because the formation of the Earth. Thorium is found jointly along with lanthanides within the phosphate mineral monazite (LnPO4), and uranium takes place like pitchblende U3O8 and carnotite K2(UO2)2(VO4)2.3H2O. Uranium is mainly used like a nuclear fuel, like the isotope 235U goes through neutron-induced fission, the nucleus divided into two small fragments together with more neutrons that can thus start a chain reaction. The energy liberated (about 2×1010 kJ mol-1) is very much greater than that is obtainable from chemical reactions.
The first members of radioactive decay series are 232Th, 235U and 238U are, creating other radioactive elements along with atomic numbers 84-91 that are therefore exist in small amounts in thorium and uranium ores. The 238U series is demonstrate in Topic A, diagram 1. Every series ends with a dissimilar stable isotope of lead (208Pb, 207Pb and 206Pb, correspondingly) and the proportions of these exist in natural lead samples changes detectably. This ariation can be employed to provide geological information, that is including an estimate of the age of the Earth.
Transuranium elements across U do not take place naturally on Earth but can be prepared artificially. The neutron irradiation of 238U within nuclear reactors gives 239U, that is quickly goes through β decay to 239Np and thence to 239Pu.
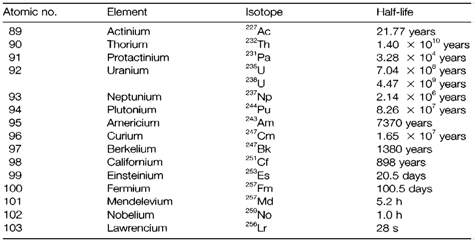
Additional neutron irradiation makes heavier actinides in increasingly smaller amounts, up to Fm. The remaining elements Md, No and Lr not obtained in this method but have been created in exceedingly small quantities through bombardment of lighter actinides with nuclei like 4He and 12C using particle accelerators. (Note: the longest lived isotopes that are listed in Table 1 are not essentially the ones most easily made.) Similar techniques have been employed to create transactinide elements with atomic number up to 110, most probably forming part of a 6d transition series. Though, the very small quantities made (frequently some atoms only) and their extremely short half-lives make chemical studies almost not possible.