Chemical properties
Different from the 4f orbitals in the lanthanides, the 5f orbitals within the earlier actinide elements are more extended and so can be occupied in chemical bonding. This directs to a pattern of chemistry more similar to that found in the d block, with the opportunity of variable oxidation states up to the maximum probable determined through the number of valence electrons. Most thorium compounds consist of ThIV (example ThO2) and with uranium the states from +3 to +6 can be created. UO2 is often nonstoichiometric, and the natural mineral U3O8 possibly consists of UIV and UVI. Uranium hexafluoride is created industrially by using ClF3 like a fluorinating agent:
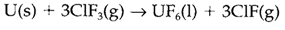
Being unpredictable, it is employed to separate the isotopes 235U and 238U for nuclear fuel applications. Several other UVI compounds consist of the uranyl ion UO2+2a linear unit along with bonding involving both 5f and 6d orbitals: instances involve the mineral carnotite and Cs2[UO2Cl4] in which uranyl is complexed to four chloride ions.
In the series the maximum attainable oxidation state is +7, within the mixed oxides Li5AnO6 (An=Np, Pu). High oxidation states become more strongly oxidizing with increasing atomic number, like in the d block. This tendency is demonstrated in Fig. 1. That depicts a Frost diagram with the oxidation states of a number of actinides that are found in aqueous solution. The oxocations AnO+2and AnO2+2 are feature for AnV and AnVI with An=U, Np, Pu and Am, except the slopes of the lines in the figure depict their progressively more strong oxidizing character. Complex solution equilibria are feasible: with Pu, for instance, all states from +3 to +6 can be exists simultaneously. The dissimilar redox stability of U and Pu is significant in nuclear fuel reprocessing, one function of that is to separate not used uranium from
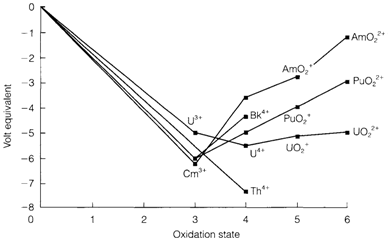
Fig. 1. Frost diagram showing the oxidation states of some actinides in aqueous solution at pH=0.
239Pu that is itself used like a nuclear fuel. Dissolving the used up fuel elements in aqueous HNO3 gives PuIV and UVI. Consequent separation steps then rely on variations in complexing power and solubility of these ions.
The organometallic chemistry is very much less wide than that of the d block, and different from that of the lanthanides through virtue of the large sizes of the early actinides and their wide variety of accessible oxidation states. Uranium has been very much investigated than other elements. Usual compounds involve the cyclopentadienyl (Cp=η5-C5H5) compounds [AnCp3], [AnCp4] and mixed Cp-halides like [AnCp3Cl]. Mainly interesting is the sandwich compound [U(η8-C8H8)2] along with two planar cyclooctatetraene rings termed as uranocene; analogs are created with neighboring actinides. Even though formally they can be considered like compounds of An4+ with the aromatic 10 π electron ring [C8H8]2- there is a few covalent bonding that is involving actinide 5f and 6d orbitals.
Later actinides depict a more restricted range of oxidation states, and are more identical to the lanthanides. The +4 state is observe in AnO2 and AnF4 so far as Cf. It becomes increasingly more oxidizing for later elements, although with a break at Bk4+ (that is more easily created than Cm4+ or Cf4+) following the half-filled 5f shell and so analogous
to the happening of Tb4+ in the lanthanides. From Am to Md the +3 state is mainly stable in aqueous solution and solid compounds. Though, near the end of the series the +2 state shows more stable than in the lanthanides and is the usual one for No. This variation must reflect a dissimilar balance of ionization energies and lattice or solvation energies, although the data needed to comprehend it in detail are not available. With only some atoms presented and along with extremely short half-lives, chemical investigations of afterwards actinides rely on tracer methods by using a stable element of supposed similar chemical behavior to act like a carrier. For instance, the existence of No2+ can be inferred from its precipitation (and subsequent detection through its radioactivity) with Ba2+ as BaSO4 under circumstances in which other oxidation states form soluble compounds.