Base Strength:
Electronegativity
Electronegativity has a significant affect to play on basic strength. If we compare the hydroxide ion, fluoride ion, amide ion and the methyl carbanion, after that the order of basicity is as shown in the figure.
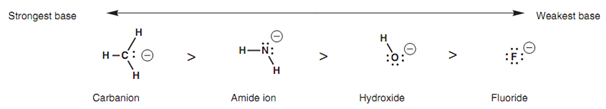
Figure: Comparison of basic strength.
The strongest base is the carbanion because this has the negative charge located on the least electronegative atom - the carbon atom. The weakest base is the ?uoride ion that has the negative charge positioned on the most electronegative atom - the ?uorine atom. Strongly electronegative atoms like ?uorine are capable to stabilize a negative charge creating the ion less reactive and less basic. The order of basicity of the anions created from alkanes, amines, and alcohols follows an identical order for similar reason shown in the figure.
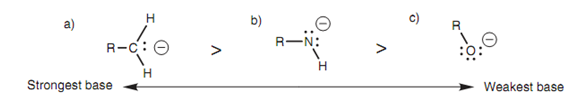
Figure: Comparison of basic strengths: (a) a carbanion; (b) an amide ion; (c) an alkoxide ion.