Inductive effects:
Inductive effects influence the strength of a charged base through influencing the negative charge. For instance, an electron-withdrawing group assists to stabilize a negative charge, resultant in a weaker base. Electron-donating group will destabilize a negative charge resultant in a stronger base. As we described earlier that while we compared the relative acidities of the chlorinated ethanoic acids Cl3CCO2H, Cl2CHCO2H, ClCH2CO2H, and CH3CO2H. Trichloroacetic acid is a strong acid because its conjugate base (the carboxylate ion) is stabilized through the three electronegative chlorine groups shown in below figure.
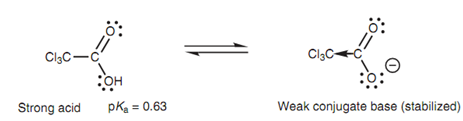
Figure: Inductive effect on the conjugate base of trichloroacetic acid.