Resonance:
The negative charge on a number of conjugate bases can be stabilized through resonance. Resonance includes the movement of valence electrons around a structure, resultant in the sharing of charge among different atoms - a process termed delocalization. The effects of resonance can be demonstrated by comparing the acidities of ethanoic acid (pKa 4.76), phenol (pKa 10.0) and ethanol (pKa 12.4). The pKa values demonstrate that ethanoic acid is a stronger acid as compared to phenol, and that phenol is a stronger acid as compared to ethanol.
The differing acidic strengths of ethanoic acid, phenol and ethanol can be described through considering the relative stabilities of their conjugate bases shown in figure.
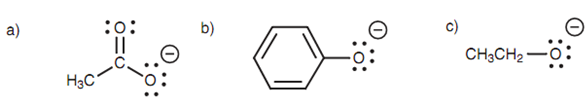
Figure: Conjugate bases of (a) ethanoic acid; (b) phenol; (c) ethanol.
The charge of the carboxylate ion is on an oxygen atom, and because oxygen is electronegative, the charge is stabilized. Though, the charge can be shared along with the other oxygen leading to delocalization of the charge. This occurs through a resonance interaction among a lone pair of electrons on the negatively charged oxygen and the π electrons of the carbonyl group shown in figure. A lone pair of electrons on the 'bottom' oxygen creates a new π bond to the neighboring carbon. At similar time as this occurs, the weak π bond of the carbonyl group breaks. This is necessary or else the carbonyl carbon would end up with 5 bonds and that is not allowed.