Inductive effects:
Fluorine atoms are much strongly electronegative and the meaning of this is that each C-F bond is strongly polarized like that the carbon bearing the fluorine atoms turns into strongly electropositive. Because this carbon atom is now electron deficient, it will 'demand' a great share of the electrons in the neighboring C-C bond. This results in electrons being withdrawn from the neighboring carbon, creating it electron deficient also. This inductive effect will carry on to be felt by the several bonds of the structure. It will decrease by the bonds but it is still important enough to be felt at the negatively charged oxygen. Because the inductive effect is electron withdrawing it will decrease the negative charge on the oxygen and assist to stabilize it. The meaning of this is that the original fluorinated alcohol will lose its proton more readily and will be a stronger acid.
This inductive effect describes the relative acidities of the chlorinated ethanoic acids Cl3CCO2H (0.63), Cl2CHCO2H (1.26), ClCH2CO2H (2.87), and CH3CO2H (4.76). Trichloroethanoic acid is the strongest acid because its conjugate base (the carboxylate ion) is stabilized through the inductive effect formed by 3 electronegative chlorine atoms. Since, the number of chlorine atoms decrease, thus the inductive effect. Inductive effects as well describe the variation among the acid strengths of ethylamine (pKa ~40) and ammonia (pKa ~ 33). The pKa values illustrate that ammonia is a stronger acid as compared to the ethylamine. In this case, the inductive effect is electron donating. The alkyl group of ethylamine improves the negative charge of the conjugate base and thus destabilizes it, making ethylamine a weaker acid than ammonia shown in diagram.
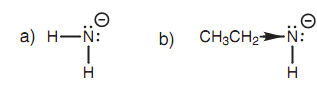
Figure: Conjugate bases of (a) ammonia and (b) ethylamine.