Electronegativity:
The acidic protons of several molecules are not evenly acidic and their relative acidity depends upon a number of factors, one of which is the electronegativity of the atom to which they are attached. For instance, consider ethanoic acid, hydro?uoric acid, and methylamine shown in the below figure. Hydro?uoric acid has the most acidic proton because the hydrogen is attached to a strongly electronegative ?uorine. The ?uorine strongly polarizes the H-F bond like that the hydrogen becomes extremely electron de?cient and is simply lost. One time the proton is lost, the ?uoride ion can stabilize the resultant negative charge.
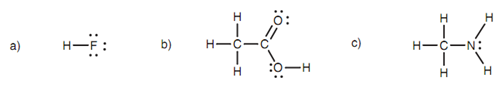
Figure: (a) Hydro?uoric acid; (b) ethanoic acid; (c) methylamine.