Separation of Hg:
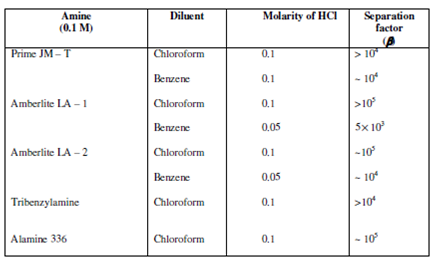
The separations are not possible in Aliquat 336 because Zn (II) and Cd (II) also show higher extractions in this amine even at lower acid concentrations. Chloroform, in general, is a better diluent than benzene for the above mentioned separations.
Table: Separation of Hg (II) from Zn (II) at 0.1 M HCl by various amines (0.1M) in chloroform and benzene
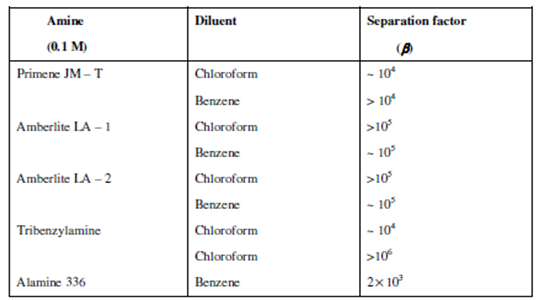
Table: Separation of Hg (II) from Cd (II) from HCl solutions by various amines (0.1 M) in chloroform and benzene
The separation of Zn (II) from Cd (II) poses problem because of similar trends in their behaviour in most of the HMWA extraction systems discussed here. However, detailed information available in different amines makes it possible to offer conditions for their separation. Zn (II) can be separated from Cd (II) by employing 0.1 M Amberlite LA - 1 / Amberlite LA- 2 solution in benzene. Cd (II) is extracted in the organic phase at 0.25 M HCl leaving behind Zn (II) in the aqueous phase. The separation factors in Amberlite LA - 1 and Amberlite LA - 2 are 2.3 × 102 and 3.8 × 102, respectively. Cd (II) can be stripped from the organic layer by washing it with 4 M HNO3.