High molecular weights amines:
Table 3.1 (b): Some important binary separations involving 3d metal ions from HCl solutions using Cyanex 92.3 (toluene)
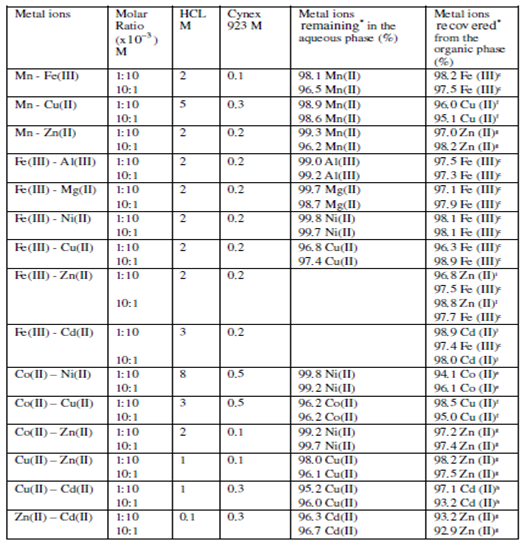
c Fe(III) stripped using 3(v) × 0.1 M H2SO4 e Co(II) stripped using 2(v) × 5 M H2SO4
f Cu(II) stripped using 2(v) × 2 M H2SO4 g Zn(II) stripped using 2(v) × 0.1 M H2SO4
h Cd(II) stripped using 3(v) × 0.1 M H2SO4 I Zn(II) stripped using 3(v) × water
j Cd(II) stripped using 3(v) × water
* An average of minimum of three determinations
Now we focus our attention to the extraction of Zn (II), Cd (II) and Hg (II) from HCl solution in different representatives of primary, secondary and tertiary high molecular weights amines and a quaternary ammonium salt for their separations.
From the extraction curves, it is apparent that mercury (II) can be separated quantitatively from Zn (II) and Cd (II) only at a very low concentration of hydrochloric acid (≤ 0.25 M). These separations can be carried out either in chloroform or benzene solutions of Prime JM - T, Amberlite LA - 1 and Amberlite LA - 2 or chloroform solutions of tribenzylamine and Alamine 336. The best conditions for the separation of Hg (II) from Zn (II) and Cd (II) in these systems are given in Table(a) and (b). The efficiency of separations, for comparison, is indicated by the separation factors given therein.
In all the above mentioned systems, Hg (II) is quantitatively extracted in the organic phase leaving behind zinc and cadmium in the aqueous solution. The Hg (II) of the organic phase can be stripped using 4 M or higher concentration of nitric acid.