Binary separations:
The separations of some binary mixtures containing Mn (II) were achieved at an appropriate molarity of HCl wherein Mn (II) is left in the aqueous phase and the other metal ion is transferred to the organic phase. The constituents of binary mixtures containing Fe (III) in combination with Al (III) / Mg (II) / Ni (II) / Cu (II) Zn (II) / Cd (II) were isolated by selectively extracting Fe (III) at conditions indicated in the table. Co (II) was separated from Ni (II) by selectively extracting it at 8 M HCl whereas for its isolation from Cu (II) / Zn (II), it was made to remain in the aqueous phase. The components of Cu (II)- Zn (II) and Cu (II) -Cd (II) mixtures were separated by selectively extracting the second one from 2 M HCl. Zn (II) and Cd (II) were isolated by subjecting their aqueous mixtures to extraction using 0.1 M HCl. At this acid concentration, Zn (II) gets extracted while Cd (II) remains in the aqueous layer.
Table 3.1 (a): Some important binary separations involving 3d metal ions from HCl solutions using Cyanex 92.3 (toluene)
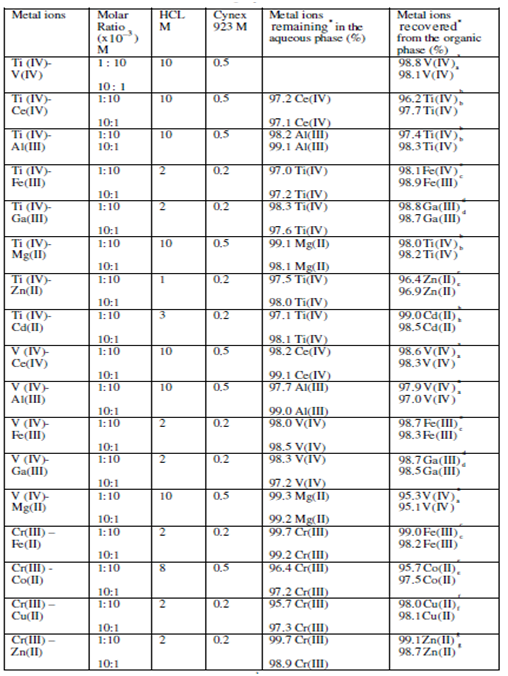
a V (IV) stripped using 3(v) × 10 M H2SO4 b Ti(IV) stripped using 3(v) × 2 M HCl
c Fe (III) stripped using 3(v) × 0.1 M H2SO4 d Ga (III) stripped using 2(v) × 0.5 M H2SO4
e Co(II) stripped using 2(v) × 5 M H2SO4 f Cu (II) stripped using 2(v) × 2 M H2SO4
g Zn(II) stripped using 2(v) × 0.1 MH2SO4 h Cd(II) stripped using 3(v) × 0.1 M H2SO4
* An average of minimum of three determinations