Molecular Absorption:
Absorption by molecules is more complex than by atoms, as there are a large number of energy states possible in case of molecules. You would recall that within case of molecules the vibration and rotation motion is also quantised in addition to electronic motion. A molecular energy level in fact has three components and its energy can be given by the following expression.
E = Eelectronic + Evibrational + Erotational
Accordingly, three types of quantised transitions occur on excitation with radiations in UV-VIS, IR and microwave regions respectively. The ultraviolet and visible radiations cause transition of electrons from the low energy electronic states to the higher energy states. Instead, IR and microwave radiations cause transitions amongst the vibrational and rotational energy levels, correspondingly.
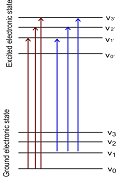
Figure: Simplified energy level diagram showing the reason for observing band spectrum in the molecules