Absorption spectrum:
Let us see what happens when a polychromatic radiation is made to impinge upon a sample containing atoms or molecules. One simple possibility is that it gets transmitted unaffected through the sample. The other possibility is the radiation coming out of the sample is devoid of the certain frequencies. In the latter case the missing frequencies are said to be absorbed by the sample. Only those photons are absorbed whose energy matches with the difference in energy of any two energy states of the sample provided the transition amongst these levels is allowed. Figure shows that the polychromatic radiation from a source when dispersed with a prism or any appropriate device, it provides a continuous spectrum of EM radiation. When the radiation is passed through a sample before being dispersed as in Figure, certain frequencies are found to be missing. These are absorbed through the sample. The spectrum so acquired is known as an absorption spectrum.
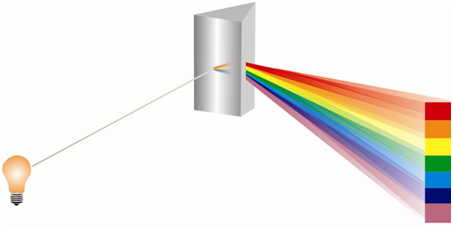
Figure: Dispersion of a polychromatic radiation through a prism to give a continuous spectrum
As the difference in the energy stages is unique for each species, knowledge of the frequencies absorbed through a given species might provide data about the species being studied. The absorption features of a given species are described in terms of the absorption spectrum that is a plot of the extent of absorption through the species as a function of wavelength or frequency or wave number of the radiation absorbed. A nature of spectrum acquired depends on the nature of the absorbing species. Let us take up the absorption of radiation through atomic and molecular species.