Oxidation states
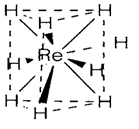
Table 1 depicts the main binary oxides and halides formed through transition elements of the 4d and 5d series. Comparison with the subsequent information for the 3d series depicts an identical pattern, with early elements in the series creating states up to the group maximum (ZrIV, NbV, etc.) in which all valence electrons are included in bonding. The main difference is that this tendency perseveres further in the lower series, the compounds RuO4 and OsO4 containing no counterpart with the 3d element iron. Following group 8, the maximum oxidation state displayed in Table 1 remains higher than ones in 3d elements, like in RuF6, IrF6, PtF6 and AuF5. As well as oxides and halides, high oxidation states are occasionally found with surprising ligands, like in the ion [ReH9]2- (1) that is formally a hydride complex of ReVII.
A complement to the stability of higher oxidation states is that lower ones (+2, +3) are less frequently found than in the 3d series.
The patterns of 4d and 5d behavior are very identical that the subsequent elements (Zr, Hf, etc.) are placed jointly in Table 1 for the earlier groups. But in later groups high oxidation states comes to be slowly less stable in the 4d compared along with the 5d series. This trend is particularly marked with Pd, Pt, Ag and Au.The slow divergence among 4d and 5d series come into existence because increasing nuclear charge beyond the series has more influence on ionization energies of 4d orbitals than on the larger 5d.
Other trend noticeable from Table 1 is the preponderance of oxidation states along with even rather than odd electron configurations in later groups; these involve PtIV, AuV (d6), PdII, AuIII (d8) and AgI (d10). AgF2 (d9) is an exception even though sometimes the stoichiometry is ambiguous, AgO being a mixed valency compound AgIAgIIIO2. Even electron configurations are favored through the large ligand field splitting observe in these series, giving low spin states, along with the d6 octahedral and d8 square-planar arrangements being specifically favorable.