Aqueous chemistry
Different from the elements of the 3d series, 4d and 5d elements have slight simple aqueous cationic chemistry. The major exceptions are Y3+ and La3+, and Ag+ that creates some soluble salts (AgF, AgNO3). The aqua Ag+ ion depicts strong class b complexing behavior, along with an affinity for ligands like NH3, I- and CN- comparable with Cd2+ in the next group. Other aqua cations can be prepared, but they are widely hydrolyzed and polymerized (example Zr4+, Hf4+), strongly reducing (example Mo3+), or have a extremely high affinity for other ligands (example Pd2+) and are hard to prepare in uncomplexed form.
Table 1. A selection of oxides and halides of elements from the 4d and 5d series. M represents either of the two elements from the corresponding group, and X any halogen unless exceptions are specified.
Several complexes are, through, formed; the most stable with early groups being ones along with F- and oxygen donor ligands and in afterwards groups ones with softer ligands like heavy halides and nitrogen donors. This tendency is identical to that found in the 3d series but is more marked. The most usually encountered solution species for later elements are chloride complexes like [PdCl4]2-, [PtCl6]4- and [AuCl4]-.
Oxoanions are created through elements of groups 5-8, instances being MoO2-4, ReO-4and RuO2-4 . They are always less strongly oxidizing than their counter-parts in the 3d series. MoVI andWVI, and to a lesser extent NbV and TaV, form wide series of polymeric oxoanions: isopolymetallates like [Mo6O19]2- and [Ta6O19]8- are mainly based on metal oxygen octahedra sharing edges and corners; heteropolymetallates like the phosphopolymolybdate ion [PMo12O40]3- include other elements, in this example like a tetrahedral PO4 group.Solid structures Larger ionic radii as compared with the 3d series elements frequently lead to higher coordination numbers. ZrO2 and HfO2 can take on the eight-coordinate fluorite structure with an exclusive seven-coordinate structure termed as baddeleyite (cf. TiO2, rutile). Of a structure with six-coordination ReO3 is the prototype, and is accepted also
(in little distorted form) through WO3, in difference to CrVI, that is tetrahedral. MoO3 and WO3 form wide series of insertion compounds termed as oxide bronzes. In halides, higher coordination frequently leads to polymeric forms for compounds MX4 and MX5 in which the subsequent 3d compounds are molecular.
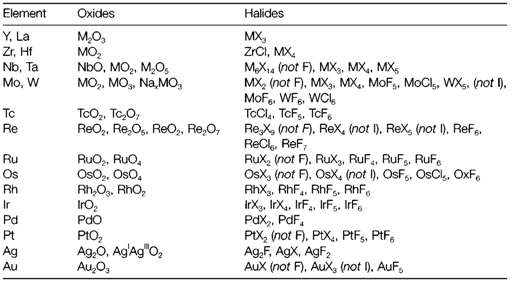
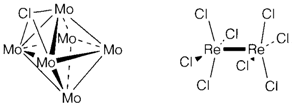
In low oxidation states compounds of elements extremely often have extensive metal-metal bonding. Occasionally this acts to alter an otherwise normal structure, like in NbO2, MoO2 and WO2, that have the rutile form distorted through the formation of pairs of metal atoms. Frequently the structures are exclusive. For instance, MoCl2 consist of [Mo6Cl8]4+ clusters formed through metal-metal bonded octahedral along with chlorine in the face positions. Complex halides frequently show metal-metal bonding, like in [Re2Cl8]2- (3) in which all four d electrons of ReIII are paired to create a quadruple bond.
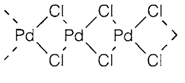
Later elements are tends to depict coordination geometries that are particular to specific low-spin electron configurations. d6 compounds are always octahedral, d8 almost all the time square planar (example in PdCl2 4 and PdO; a uncommon exception is PdF2 that like NiF2, have the octahedral rutile structure along with two unpaired electrons per Pd). The d10 configuration frequently has a trend to linear two-coordination (cf. HgII, Topic G4). Even though AgF, AgCl and AgBr have the rocksalt structure another AgI compounds like Ag2O contain two-coordination, and it is general for AuI; for instance, AuCl has a chain structure along with a linear Cl-Au-Cl arrangement.