Oxidation States
Table 1 depicts some oxides and halides of the 3d series elements that are selected to depict the range of stable oxidation states. These follow similar trends as found in aqueous chemistry. Elements early on in the series create compounds up to the group oxidation state, for instance, CrO3, TiO2, and VF5. With increasing group number the higher oxidation states become increasingly difficult to form, and can be notice only with oxides and/or fluorides, and occasionally only in ternary but not binary compounds. For instance, with VV we can create VF5 and V2O5 but not VCl5.With MnVII the single binary compound is Mn2O7 but it is much less stable than ternary permanganates like KMnO4.
The stabilization of high oxidation states through O and F might be attributed as a minimum partly to their small size that gives the large lattice energies essential according to the ionic model to compensate for ionization energies. Further lattice stabilization is probable in ternary structures, such as in compounds like K2FeO4 and K2CoF6 in which no binary compounds with the subsequent oxidation state are stable. It should be identified that several of the compounds in high oxidation states are not extremely ionic, and arguments that are based on the high bond strengths formed through O and F to more electropositive elements might be more satisfactory than using the ionic model.
Table 1. A selection of oxides and halides of the elements Sc-Cu. X represents any halogen except specified. Oxidation states are shown in mixedvalency and ternary compounds.
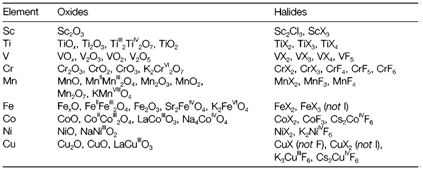
Low oxidation states (example +2) are of limited stability for the early elements. The strange metal-rich compound Sc2Cl3 has a structure with wide Sc-Sc bonds. Compounds like TiOx and VOx are nonstoichiometric and are also stabilized through metal-metal bonding by using d electrons. With Cu the +1 oxidation state is stable with in compounds like Cu2O and CuCl, but CuF is not recognized, most probably since the larger lattice energy of fluorides creates this unstable regarding to disproportionation to Cu and CuF2. The differential stability of the oxidation states with dissimilar halogens is also displayed through the presence of CuI but not CuI2.
The presence of various stable oxidation states provides rise to the possibility of mixed valency compounds in which an element is exist in different oxidation states. So the compounds M3O4 with M=Mn, Fe, Co, have both MII and MIII states exist. Several oxides also depict nonstoichiometry in which a continuous range of composition is probable. For instance, 'TiO' is really TiOx in which x can change continuously over a broad range, and 'FeO' does not in fact exist but is about Fe0.9O (and thermodynamically unstable below 550°C). Such types of nonstoichiometric compounds are better explained through the phase diagrams than through simple stoichiometric formulae that can be misleading.