Oxidation states
Diagram 1 depicts a Frost diagram for the elements Sc-Zn along with the electrode potentials for aqueous species suitable to acid solution (pH=0). Lines along with positive (negative) slopes in this figure indicate potentials that are more (less) strongly oxidizing regarding the standard couple (H+/H2). The figure shows clearly the major trend to less electropositive character beyond the series.
For the M2+/M or M3+/M couples the negative slopes depict that metals early in the series are strong reducing agents. This trend decreases beyond the series, and copper has a positive Cu2+/Cu slope, depicting that copper metal does not react with acids to provide hydrogen. Though, it will dissolve in strongly oxidizing acids like HNO3 or in the existence of some complexing agents.
Higher oxidation states are also very much accessible for elements early on in the series. so far as Mn (group 7), elements can reach the group oxidation state (subsequent to the formal d0 electron configuration). This come to be more oxidizing along the series TiIV<VV<CrVI<MnVII, and permanganate MnO4- is used extensively like a strong oxidizing agent in acid solution. For all elements M2+ ions are stable apart from Sc and Ti, in which these oxidation states are very strongly reducing to present in water. M3+ ions are created through elements up to Co, even though for Mn and Co the uncomplexed ions are too strongly oxidizing in acid solution. The ionization energy (IE) tendency is the very significant issue that controlling the change in redox stability and the discontinuity in the tendency of third IE values after Mn is reflected in an identical
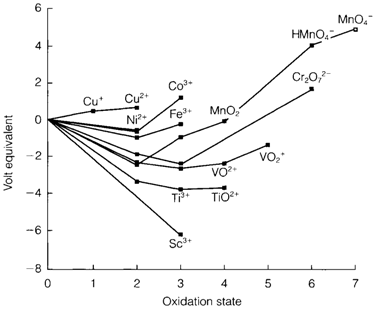
Fig. 1. Frost diagram for elements of the 3d series in aqueous solution at pH=0.
Break with in the M3+/M2+ redox potentials, Fe3+ being less strongly oxidizing than Mn3+. Changing solvation energies also comprise an influence, and these are in turn affected through ligand field stabilization energies. The Cr3+/Cr2+ couple is reducing more than supposed from its third IE like a consequence of the large reduction of LFSE among Cr3+(d3) and Cr2+ (d4). Also seen in the diagram is that a number of intermediate oxidation states are prone to disproportionation. So MnVI goes through the following reaction in acid solution:
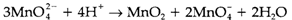
Mn3+ and Cu+ also disproportionate even though all these reactions can be affected through pH or complexing.