Effect of pH
In a half-cell reaction like:
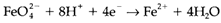
rising pH (and hence decreasing H+ concentration) will favor the left side and so lower the redox potential. So some high oxidation states like FeO2-4are much more accessible in alkaline than in acid solution. Changing pH can also change the trend to disproportionate. For instance, MnIII, that is not stable in alkaline solution, is yet readily created as Mn(OH)3 through air oxidation of Mn(OH)2. MnVI also resists disproportionation with in alkaline solution.
The species exist may change with pH in a way that relies on the oxidation state. Low oxidation states (+2) are all the time cationic and like pH increases an insoluble hydroxide is ultimately precipitated. Like the oxidation state get increases so does the acidic character of the hydrated cation. Thus M3+ ions go through protolysis even at pH values as low as 1 or 2; deprotonation can be a first step in the creation of oxygen-bridged dimers like with
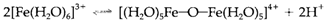
These may go through further polymerization before precipitating like Fe(OH)3.
High oxidation states (+6, +7) are acidic and all the time exist as anionic species(CrO2-4, MnO-4) even though with CrO2-4dimerization takes place at low pH:
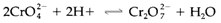
With intermediate oxidation states very much complex amphoteric and polymeric nature is observed. So VV creates hydrated VO+2 in acid solution below pH 2, and the anionic species VO3-4 at high pH. Over an intermediate pH range complex polyvanadates are created, very prominent being the decavanadate ion [V10O28]6- (generally exist in protonated forms).