Complex formation
Complexing behavior relies on the oxidation position and state in the series. Higher oxidation states are likely to form stronger complexes along with the 'hard' anionic ligands F- and chelating agents like EDTA. So we have complexes like [TiF6]2-, [VF6]- and [FeF6]3-. Later elements, particularly in low oxidation states, contain more affinity for softer ligands like heavier halides or ammonia. The stabilities of complexes observe with several ligands, particularly ammonia or amines, follow a trend termed as the Irving-Williams series:
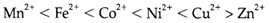
For this trend there are two contributions
(a) the usual decrease in electropositive character resultant from increased effective nuclear charge and
(b) ligand field stabilization energies that get increases the stability of complexes with ligands higher with in the spectrochemical series than water in all ions apart from Mn2+ (d5) and Zn2+ (d10).
Complexing can have a strong influence on redox chemistry, the common rule being that a ligand stabilizes any oxidation state it complexes with most strongly. Two significant instances are the following.
(i) Cu+ cretaes strong complexes with ligands like CN- and I- so that the CuI/Cu potential come to be negative and copper metal will react along with the acids to create hydrogen; these types of ligands also stabilize the CuI state against disproportionation.
(ii) Several ligands (example NH3) complex strongly with Co3+, providing a low-spin d6 state with a large LFSE. The resultant complexes like [Co(NH3)6]3+ are less strongly oxidizing than aqua Co3+ ion, that itself oxidizes water.
Commonly speaking, that negatively charged ligands complex very more strongly with ions of higher oxidation state and thus reduce the redox potential, where neutral π-acceptor ligands, being electron withdrawing, be likely to stabilize cations of lower charge and so increase the potential. Ligand field stabilization and other influences cause several complications, though, that can upset these simple generalizations.