One way to create ethanol for use as a gasoline additive is the reaction of water vapor with ethylene:
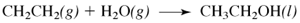
a. write this equation using Lewis structures.
b. Is this reaction exothermic or endothermic?
c. In your calculation, was it essential to break all the chemical bonds in the reactants to form the product ethanol? Illustrate your answer.
a. 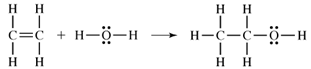
b. Bonds broken in the reactants:
1 mole C-to-C double bond = 1(598 kJ) = 598 kJ
1 mole O-to-H single bond = 1(467 kJ) = 467 kJ
Total energy absorbed in breaking bonds =1065 kJ
Bonds formed in the products:
1 mole C-to-C single bond = 1(356 kJ) = 356 kJ
1 mole C-to-H single bond = 1(416 kJ) = 416 kJ
1 mole C-to-O single bond = 1(336 kJ) = 336 kJ
Total energy released in forming bonds =1108 kJ
Total energy change: (1065 kJ) + (-1108 kJ) = - 43 kJ
Because the energy released in forming bonds is bigger than the energy absorbed in breaking bonds, the net energy change is negative and the overall reaction is exothermic.
c. No, it is not essential to break all of the bonds. There are four carbon-to-hydrogen single bonds on the reactant side, and they are also in the product, ethanol. One of the oxygen-to-hydrogen bonds in water remains intact in the product.