Q. Write electron configuration for a neutral atom of silicon?
Writing Electron Configurations by means of Noble-Gas Notation Use noble-gas notation to write the electron configuration for a neutral atom of silicon.
To start find silicon on the periodic table and locate the preceding noble gas Silicon (Si) is atomic number 14 and the preceding noble gas is neon (Ne) atomic number 10.
Begin the electron configuration by writing the symbol [Ne]. Note that [Ne] restore 1s22s22p6 which is neon's electron configuration.
After that note that silicon has four more electrons than neon. As-per to the energy sublevel diagram two of these electrons will fill the 3s sublevel and the remaining two electrons will occupy orbitals in the 3p sublevel.
By combining this information you are able to write the following electron configuration for silicon.
Si [Ne]3s23p2
Valence electrons when elements combine chemically merely the electrons in the highest principal energy level of each atom are involved. Consequently these outermost electrons called valence electrons determine most of the chemical properties of an element.
Later in your chemistry course you will learn the way in which elements form chemical bonds. For the reason that bonding involves an atom's valence electrons it is useful to can sketch a representation of an element's valence electrons. The American chemist G. N. Lewis planned the electron-dot structure to show an atom's valence electrons by writing dots around the symbol of the element.
In writing electron-dot structures a sole dot is used to represent each valence electron. One dot is placed on each one of the four sides around the symbol before any two dots are paired together.
The following instance illustrates the process. The electron-dot formation for hydrogen which has one electron is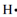 . The electron dot structure for helium 1s2 is
. The electron dot structure for helium 1s2 is 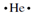 the electron configuration of lithium is 1s22s1 except the two 1s electrons are in a stable inner energy level and don't participate in chemical changes. Only the outmost 2s electron is a valence electron so the electron-dot structure for lithium is
the electron configuration of lithium is 1s22s1 except the two 1s electrons are in a stable inner energy level and don't participate in chemical changes. Only the outmost 2s electron is a valence electron so the electron-dot structure for lithium is 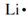 . The electron formation of beryllium is 1s22s2 but merely the two 2s electrons are valence electrons so the electron-dot structure for beryllium is
. The electron formation of beryllium is 1s22s2 but merely the two 2s electrons are valence electrons so the electron-dot structure for beryllium is 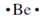 the electron-dot structure for boron with three electrons in the second energy level (1s22s22p1) is
the electron-dot structure for boron with three electrons in the second energy level (1s22s22p1) is 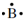 . The electron formation of oxygen is 1s22s22p4 and its electron-dot structure is
. The electron formation of oxygen is 1s22s22p4 and its electron-dot structure is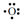 . A fresh principal energy level begins with sodium whose electron configuration is 1s22s22p63s1. Sodium's electron-dot structure is
. A fresh principal energy level begins with sodium whose electron configuration is 1s22s22p63s1. Sodium's electron-dot structure is 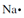 for the reason that merely the 3s electron is a valence electron. The other electrons are in inner energy levels.
for the reason that merely the 3s electron is a valence electron. The other electrons are in inner energy levels.