Q. What happens to the energy values of d- orbitals?
Let us see what happens to the energy values of d- orbitals under these stages. We start with the energy level diagram of a lower triplet (t2J and upper doublet (eg) in an octahedral field. Fig. When the two Trans ligands placed along pay, z-axis, are moved away the most affected orbital would be dz2. The repulsion between the orbital and the ligand will decrease, thus making it more stable compared to others. At the same time all other orbitals containing z-axis namely dxy, dyz will also be affected by the movement of the two ligands - along the z-axis. However, these will experience less effect than the dz2 orbital. These two orbitals will become lower in energy by equal amount but less than dz2 as indicated by different slopes in Fig.
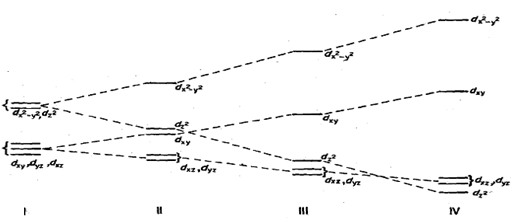
For eg set of orbitals, if d2 orbital lowered energy due to distortion, then dx2 -y2 must go ~p in energy by an equal amount 40 that the net result is no change. Similarly, dxz and dyz are lowered whereas dxy is raised in energy by such an amount that the net resultant is zero. Thus in a distorted octahedral molecule the orbitals in order of Increasing energy or decreasing stability are : dxz, dyz)- (degenerate), GI dxy, dz2 - y2 as shown in Fig. (113. A further distortion results in the formation of a square planar complex. Here, dz2 is lowered so much SO that it is almost equal to dxy, dyz orbitals Fig. (111). Much higher will be dxy and the least stable or the highest in energy would be d,2 - )2. In a few rare instances dz2 falls even below d,, and d,, orbitals Fig. (IV). We realise that in case of a square planar complex we shall not be able to calculate quantitatively the energy values of the orbitals in terms of crystal field splitting parameter (4). The actual values have. However, been calculated; based on these values we can calculate CFSE as well as the magnetic moment for any particular complex. It has been observed that at least the first row transition elements show greater tendency to form square planar rather than tetrahedral complexes. These observations are in line with the CFSE calculations for the two geometries.
We have thus come to the stage where we can appreciate that a simple electrostatic approach can lead to many useful results and these results are pretty close to the experimental values. We do not expect to get perfect results since our assumptions so far are imperfect. We are, thus, left with two choices either to discard the theory completely and look for a new approach or to modify it in such way so as to account for the observed discrepancies.