Q. What are Trioxides?
The selenium and tellurium trioxides are not stable, SO2, is the only important trioxide in the group. Its preparation has been described above.
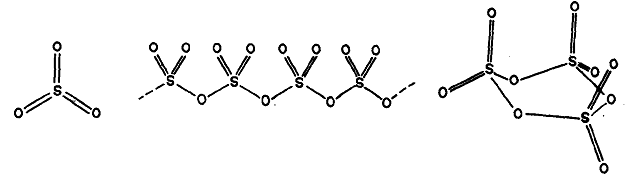
Sulphur trioxide exists in several polymorphic forms. The solid form, m .p. 290 K has a trimetric cyclic structure, in which four oxygen atoms are arranged approximately tetrahedrally around each sulphur atom, Fig. (c). This form gradually changes into a linear polymerised structure in the presence of moisture. The latter has a fibrous needle-like appearance, Fig.(b). On heating, the polymeric form dissociates into discrete SO2, molecules present in the vapour. These have a triangular, symmetrical and planar structure, Fig. (a).
SO2, is a powerful acidic oxide, it fumes in moist air and reacts explosively with water to form sulphuric acid.
SO3+ H2O------------> H2SO4
With excess SO3 H2S04 gives pyrosulphuric acid or oleum
H2SO4+ SO3-----------------> H2S2O7 (oleum)
In some reactions it acts as an oxidising agent, e.g., it oxidises HBr to free bromine.
2HBr+ SO3------------------> H2O + Br2+ SO2