Describe Common elements that make up the minerals of the Earth's crust?
The Earth's crust, which is the layer of the Earth we are on now, is made of numerous minerals and rocks. Since minerals are made of elements, it is important to know the percentage breakdown of the most common elements found in the crust. Please look at the chart below to see a percentage breakdown of the 8 most common elements in the Earth's crust.
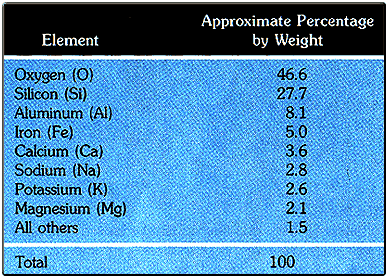
Oxygen is by far the most abundant element in the crust. Silicon, with 27.7% of the mass, combines with oxygen in many minerals. The combination of oxygen and silicon in a structure known as the silicon-oxygen tetrahedron is the molecular basis for most of the minerals in the crust. The largest mineral group, known as the silicates, features minerals that are composed of silicon and oxygen. The image below shows the formula and structure of the silicon-oxygen tetrahedron that makes up the structure of silica.
This silica structure is the heart of many more complex silicate minerals. The other atoms bond ionically or covalently to this structure to give many combinations of crystal formulas. Glass, sandstone, and many other examples of silicates are common and easy to find almost anywhere.
Carbonate minerals are similar to silicates in their basic structure. In carbonate minerals, one carbon atom bonds with three oxygen atoms, which in turn bond to many other combinations of elements. The image below shows the typical structure of a carbonate mineral.
Notice in the diagram above that the charge of 2- is very strong. This means that the carbon atom wants to bond with other, positively-charged, elements or molecules to make mineral crystals. Carbonates are the second most prolific mineral group after silicates. Limestone is a common rock that is made of these types of minerals.
There are many minerals that are neither silicate of carbonate in nature. Halite, or rock salt, is a common example we have mentioned that is a mineral of neither silica nor carbonate composition.